CDC
Now that there are authorized and recommended COVID-19 vaccines in the United States, accurate vaccine information is critical.
ECDC
1. What is SARS-CoV-2? What is COVID-19?
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is the name given to the 2019 novel coronavirus. COVID-19 is the name given to the disease associated with the virus. SARS-CoV-2 is a new strain of coronavirus that has not been previously identified in humans.
2. Where do coronaviruses come from?
Coronaviruses are viruses that circulate among animals. Some coronaviruses can infect humans.
Bats are considered natural hosts of these viruses, and several other species of animals are also known to act as sources. For instance, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is transmitted to humans from camels, while Severe Acute Respiratory Syndrome Coronavirus-1 (SARS-CoV-1) is transmitted to humans from civet cats. More information on coronaviruses can be found in ECDC’s factsheet.
Johns Hopkins
As Vaccines Rollout, Should Age, Health Conditions Be Prioritized? An Epidemiologist Weighs In
The NYT
Health experts double down on their advice for slowing the spread of the coronavirus.
BBC Future
Understanding what is driving new variants of the coronavirus to appear and what the changes mean will be crucial in our vaccine arms race against Covid-19.
The BMJ
|
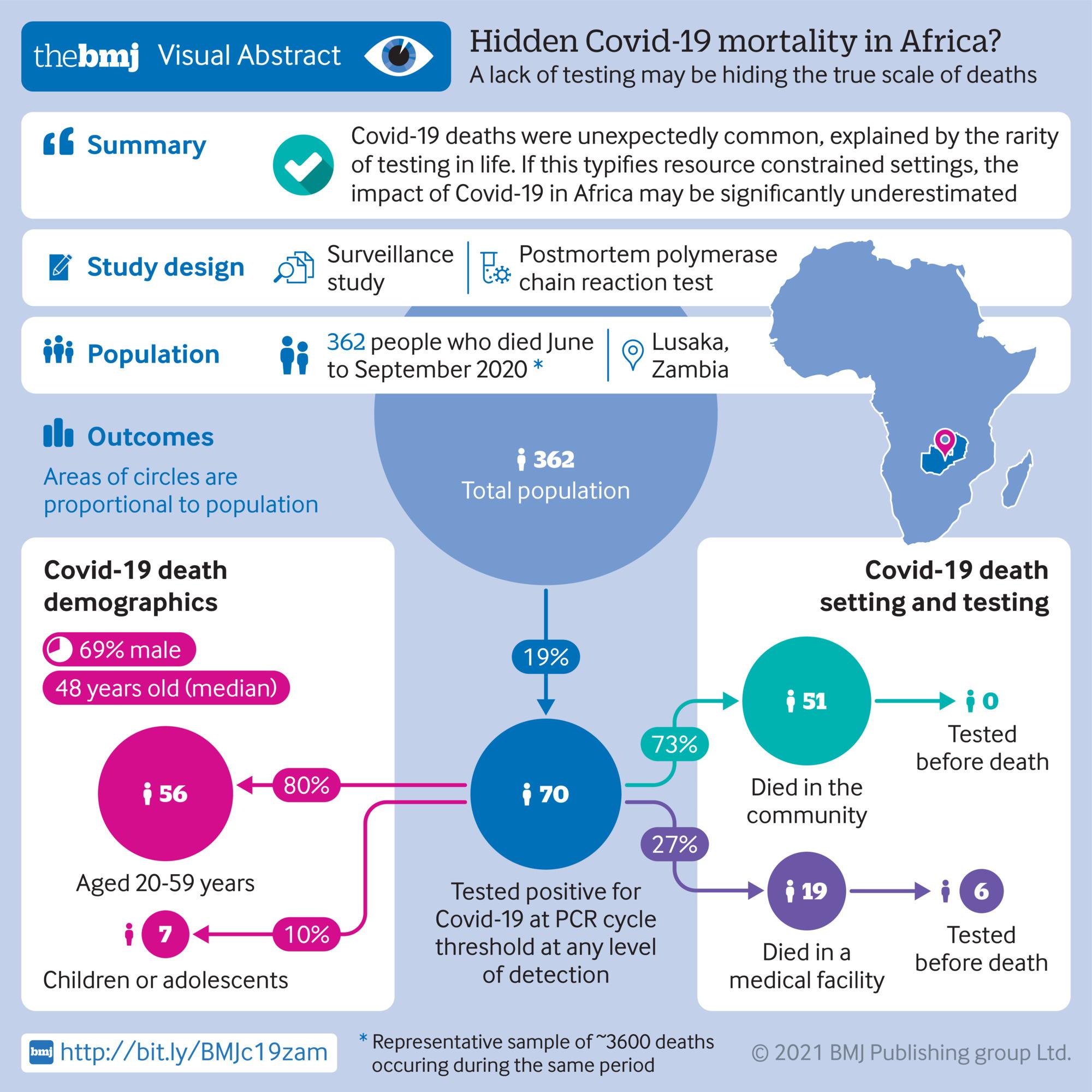
Covid-19 deaths in Africa: prospective systematic postmortem surveillance study |
The Lancet
By mid March, 2021, vaccination against COVID-19 using the ChAdOx1 nCoV-19 (AZD1222) vaccine from Oxford–AstraZeneca was paused in a number of European countries due to reports of thromboembolic events in vaccinated individuals.
According to the European Medicines Agency (EMA), 30 cases of thromboembolic events (predominantly venous) had been reported by March 10, 2021, among the approximately 5 million recipients of the Oxford–AstraZeneca COVID-19 vaccine in the European Economic Area.
The EMA subsequently stated that “The number of thromboembolic events in vaccinated people is no higher than the number seen in the general population”.
To inform the ongoing discussion on the safety of the Oxford–AstraZeneca COVID-19 vaccine, we analysed nationwide population-based data from Denmark to estimate the natural incidence of venous thromboembolism.
The BMJ
Wearing face coverings outside should be normalised because it may reduce transmission of SARS-CoV-2 in some situations and may encourage mask wearing indoors, where risks are greater say Babak Javid, Dirk Bassler, and Manuel B Bryant. But Muge Cevik, Zeynep Tufekci, and Stefan Baral argue that outdoor transmission contributes very little to overall infection rates and that efforts should focus on reducing indoor transmission
Science
SARS-CoV-2 vaccine rollout has coincided with the spread of variants of concern. We investigated if single dose vaccination, with or without prior infection, confers cross protective immunity to variants. We analyzed T and B cell responses after first dose vaccination with the Pfizer/BioNTech mRNA vaccine BNT162b2 in healthcare workers (HCW) followed longitudinally, with or without prior Wuhan-Hu-1 SARS-CoV-2 infection. After one dose, individuals with prior infection showed enhanced T cell immunity, antibody secreting memory B cell response to spike and neutralizing antibodies effective against B.1.1.7 and B.1.351. By comparison, HCW receiving one vaccine dose without prior infection showed reduced immunity against variants. B.1.1.7 and B.1.351 spike mutations resulted in increased, abrogated or unchanged T cell responses depending on human leukocyte antigen (HLA) polymorphisms. Single dose vaccination with BNT162b2 in the context of prior infection with a heterologous variant substantially enhances neutralizing antibody responses against variants.